Its worldwide production in 2009 was ca.34 million tonnes and it is expected that by 2016, it will exceed 40 million tonnes, which places PVC in the top three materials in terms of global production right after polypropylene (PP) and polyethylene (PE). Thirty five million tonnes of PVC are produced yearly on a global scale, with a 2% year-to-year increase in production.European PVC consumption is approximately7 million tonnes a year, which constitutes 15% of all the polymer materials used in Europe.In Poland, PVC production amounts to around250 thousand tonnes,and constitutes approximately 30% of all the polymers produced in the country.
PVC is the first synthetic thermoplastic material produced on an industrial scale.At the same time it is one of a few polymers which has such a vast array of modern applications despite having being discovered such a long time ago.Mixed with plasticisers, stabilisers, impact modifiers, fillers and others, it is processed into finished products by means of various techniques, which will be discussed in subsequent articles.It is a polymeric material, the modification of which in the synthesis stage is fairly simple due to the sensitivity of PVC’s parameter’s to change, hence its wide scope of applications.
The history of PVC
PVC is as old as polymers science itself. It is one of the oldest synthetic materials with the longest history of industrial production.The development of the production of plastics, including PVC, started in the first half of the 19th century in the laboratories of Justus von Liebig in Gießen.In as early as 1835, Henry Victor Regnault discovered that a white powder, later referred to as polyvinyl chloride, precipitated from a mixture of ethylene dichloride and an alcoholic solution of potassium hydroxide exposed to sunlight in a flask placed by the window.About 40 years later, in 1872, Baumann described the phenomenon of transformation (under the influence of sunlight) of liquid vinyl chloride enclosed in tubes into powder.The process which Baumman carried out involved exposure of the vinyl chloride-containing flasks to sunlight. This is how the first ever plastic material was made.However, may years had to pass since the first patents for the production of PVC came out. This happened in 1912 thanks to Fritz Klatte, who developed a method for the synthesis of vinyl chloride by means of adding hydrogen chloride to acetylene.At the beginning of the thirties of the 19th century, the first pilot lines for the production of polyvinyl chloride were set in motion in Germany and the USA.W.L. Semon obtained the first plasticised polyvinyl chloride by combining it with the high boiling tritolyl phosphate.World War II and the military’s demand for materials for cable insulation, as well as the development of coated materials softened with leather-imitating PVC made for the automotive industry (e.g. ICI Co. in 1941 was the first to use PVC-covered material on London’s busses) caused that in 1948, all the standard PVC polymer types, i.e. suspension, emulsion and paste PVCs, were already available.
Production and main properties
PVC production is relatively cheap.Polyvinyl chloride is resistant to corrosion and chemicals as well as to atmospheric conditions.It is smooth, does not conduct electricity, is recyclable and has low heat conductivity.Basic PVC polymer is a white powder with a softening point of approx.80oC and is resistant tohydrochloric, sulphuric and nitrogen (diluted) acids as well as other substances such as diluted sodium and potassium hydroxides, oils, water, alcohol and gasoline. It dissolves in ketones (acetone, cyclohexanone, etc.), esters, tetrahydrofuran, dioxane, pyridine, toluene, xylene, ethylene chloride, carbon disulfide, and dimethylformamide (unplasticized only in cyclohexanone and tetrahydrofuran).
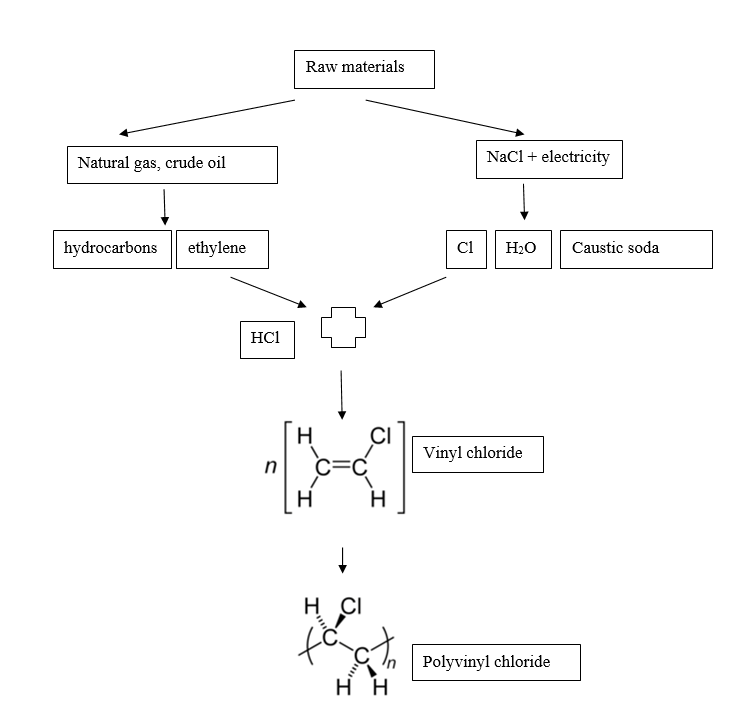
PVC production consists of 3 stages:
- Monomer synthesis,
- Polymerisation,
- Preparation of a mixture needed to obtain other products.
Various physical properties are achieved depending on the PVC polymerisation method.E-PVC obtained by means of emulsion polymerisation has the transparency and absorbability of water and electrical insulating properties which are inferior to S-PVC and M-PVC obtained by means of suspension and mass polymerisation.S-PVC and M-PVC, thanks to the way they are obtained, are very clean products.A vast majority (ca.90%) of the PVC produced is a polymer obtained via the suspension method (PVC-S).
Only 6-8% of the plastic is obtained via emulsion polymerisation (PVC-E) and is most often processed as a paste.Around two-thirds of PVC-S is then made into unplasticised, hard PVC-U, while the rest into plasticised PVC-P.
Types of PVCs and their applications
Due to the different properties of vinyl polychloride, it is used in a wide variety of applications.The main consumer of PVC is the construction industry with all its infrastructure.The construction sector constitutes two thirds of the PVC market, where it is used to make water pipes, fittings, electrical insulation, window frames, window blinds, gutters as well as wall and floor panels.
The scale of its use, especially in construction, derives primarily from the above mentioned functional and economic properties, as well as its safety and its construction traits which include longevity and resistance to destructive environmental factors such as chemicals and the elements.
| PVC-U
(unplasticised, hard) |
PVC-P
(plasticised, soft) |
| Resistant to corrosion, chemicals, oils, atmospheric factorsEasy to process, join, dye and print on, lightFire-resistant and self-quenching.Possesses ideal insulating properties and can be used to make transparent, semi-transparent and opaque products. | Oxoviflex® phthalate-free plasticizers, e.g. DEHT (Grupa Azoty), are the most effective softening additives
It is ideally transparent and flexible, resistant to tearing, Elastic and resistant to atmospheric factors, is impermeable to oxygen and smells. It is formable at a lower temperatures than hard polyvinyl chloride |
It can be hot-moulded by extrusion, pressing or injecting, as a result of which the products formed achieve:
|
The products are made in the processes of extrusion, pressing, calendring, injection and molding.Depending on the type and amount of plasticiser used, they demonstrate varying degrees of:
In order to reduce their price, they contain up to 50% filler (kaolin, chalk, silica flour) However, the properties of plastics with a filler are worse than those without one despite demonstrating the same level of hardness. |
| pipes (water),fittings,sections used in construction and the furniture industry,containers,panels,tiles,bottles,
disposable syringes, hard film. |
cable insulation,soles,synthetic leather,flooring sheets,car upholstery,wallpaper,garden film and hoses,
construction film and packaging, a wide range of products for the medical industry (bags for blood and bodily fluids, drains, catheters, speculums and others. |
Conclusion:
The industrial history of polyvinyl chloride is over 90 years old.In this period, PVC has managed to gain a strong market standing and is currently a key raw material used in the production of a variety of polyvinyl chloride materials.The range of its application is so wide that many areas of our present-day economy would no longer be able to function without PVC.A constant rise in demand for this raw material is why research is underway to develop more effective vinyl chloride polymerisation methods (VCM) and obtain PVCs with increasingly better processing and functional properties.The great importance of PVC for the economy stems primarily from the availability of raw materials, its low production costs and its functional advantages.
PVC is very easily modified, hence the properties of polyvinyl chloride materials produced from this polymer can easily be adjusted to various requirements depending on the planned end-use.Modification is achieved through proper selection of the PVC mixture’s ingredients, which will be discussed in the articles to come.